Heart Failure Treatment – AnaCardio´s Unique Solution
AnaCardio is developing novel inotropic agents with a unique mode-of-action based on the ghrelin signalling pathway, intended to increase contractility without causing adverse tachycardia, arrhythmia, ischemia, or hypotension. The agents increase cardiomyocyte contractility and force, leading to increased cardiac output which can potentially improve organ function, quality of life and functional capacity, and reduce the risk of hospitalization and death. AnaCardio´s treatment concept stems from ground-breaking research from Karolinska Institutet and Founder Professor Lars Lund.
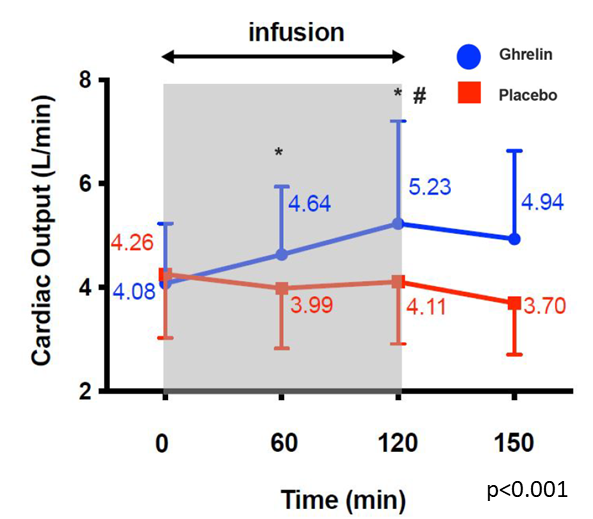
A proof-of-concept study was performed at the Karolinska University Hospital in which 30 out-patients with chronic heart failure were randomized to ghrelin or placebo given intravenously over 120 minutes. The primary outcome measure was cardiac output (CO) at 120 minutes. The ghrelin treated group increased CO significantly by 28%. This was achieved without any signs of arrythmias, tachycardia, ischemia or hypotension.
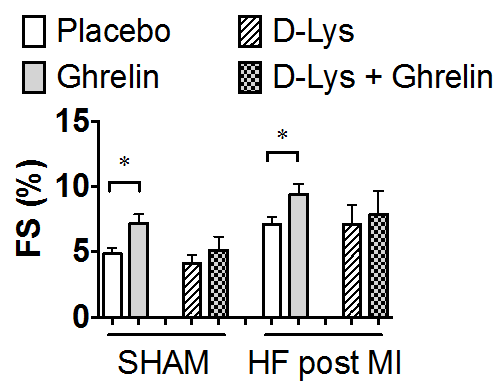
Furthermore, the ghrelin peptide increased contractility in an ex-vivo model experiment investigating beating mice cardiomyocytes. The observed increase in contractility and force was observed without elevated calcium concentrations and instead achieved by increased calcium sensitization (1).
Ghrelin peptide study achieved proof-of-concept in 30 HFrEF patients by increasing cardiac output over 120 minutes.
1. Ghrelin increases contractility in beating mouse cardiomyocytes…
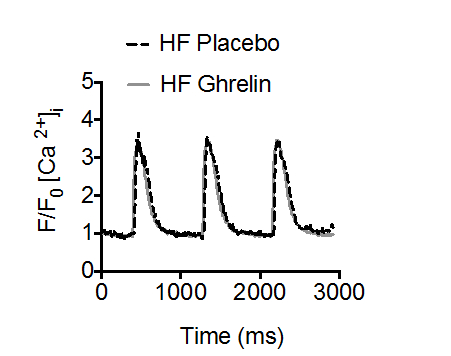
2. … without changing Ca2+ transients/concentrations.
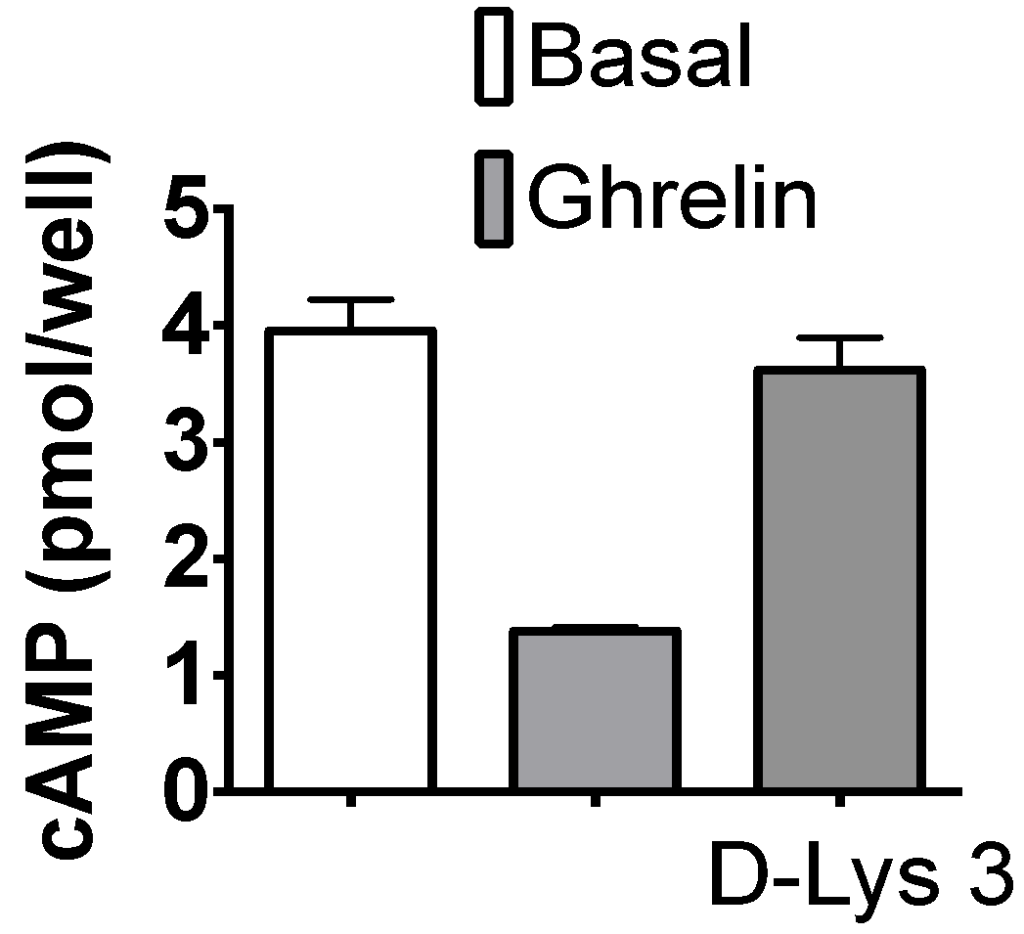
3. Ghrelin reduces cAMP levels in cardiomyocytes…
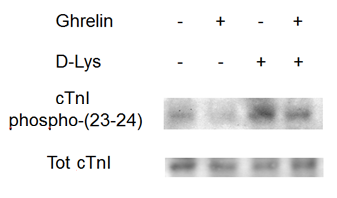
4. … and Ca2+ sensitization occurs through reduction in troponin I phosphorylation.
AC01 is an oral ghrelin peptidomimetic small-molecule – a first in-class calcium sensitizing inotrope. In preclinical studies, AC01 increases contractility/force and sensitizes cardiac cells to calcium, which is differentiated from conventional inotropes that increase calcium concentrations and flux. The latter may cause life-threatening arrythmias and cardiac ischemia. AC01 is the first agent to safely target the underlying mechanism of HFrEF by improving contractility, and as such would potentially have both direct effect on cardiac contractility and organ functions, as well as longer term disease-modifying effects.

Calcium sensitizing inotrope
Based on AC01’s mechanism of action it is viewed by Key Opinion Leaders as a “safe inotrope” that could be used chronically – a holy grail in the field.
Treaters believe that an inotrope that sensitizes cardiac cells to calcium – vs increasing calcium levels – will be safe. This is in contrast to conventional inotropes, which have a desirable effect of increasing the contraction force of the heart but have a negative effect on mortality and are only used as a last resort.

Contractility, Force & Cardiac Output
When considered in context of AC01’s expected safe adverse event profile, doctors identified AC01 as a “safe inotrope”, which would be a true disease modifying therapy that could reverse disease effects – as long as patients stay on therapy chronically.

Small Molecule, oral treatment
As an orally available small molecule, AC01 meets treater’s basic requirements for a novel agent in patients with chronic heart failure.
”The arrhythmias and ischemia we see in conventional inotropes are because of the chronically elevated Ca2+ levels that they cause. This drug will change how sarcomeres themselves react to Ca2+ – potentially avoiding safety concerns”
– HFrEF KOL
”This would be the holy grail. Patients would feel better, have improved blood flow, better system function. This is essentially a safe inotrope – and the only reason we don’t use inotropes more widely is that they worsen outcomes”
– HFrEF KOL
”For chronic patients, oral is what I’m looking for, all the other drugs I use in in these patients are oral. This is in contrast with the inotropes we have now, which we use outpatient in extreme cases and require me to set up patients with infusion pumps. This molecule is much better in many regards”
– HFrEF HVT
AnaCardio has initiated a Phase 1b/2a study in HFrEF patients:
AC01-01 Heart Failure Dose Escalation and 28 day Cohort Expansion Clinical Trial.
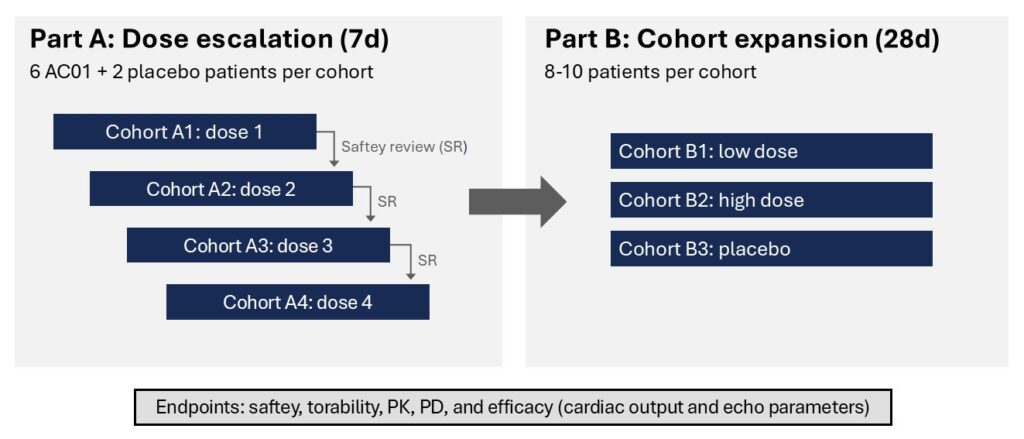
It is a randomized, double-blind, multiple ascending dose, placebo-controlled, safety, efficacy, pharmacokinetic (PK) and pharmacodynamic (PD) trial with AC01 in patients with stable heart failure with reduced ejection fraction (HFrEF). The study started in the spring 2023 and is now ongoing at selected sites in Europe.
The dose-escalation phase (Part A/Phase 1b) was completed in fall 2024. A total of 32 patients, 8 in each of 4 sequential dose cohorts, were treated with ascending doses of AC01 or placebo for 7 days.

AC01 Phase 1b/2a study design
Feel free to contact us if you have any questions or want to book a meeting.
Contact us